Regulatory compliance and product safety in international health product sales require adherence to specific legal frameworks and standards that ensure the safety, quality, and efficacy of health-related products.
Key points include:
-
Regulatory Authorities and Frameworks: Health products such as medicines, medical devices, cell therapies, cosmetics, and health supplements are regulated by dedicated authorities that enforce laws like the Health Products Act and Medicines Act. These authorities oversee product registration, licensing, and compliance with safety standards to protect consumers.
-
Product Registration and Licensing: Dealers or manufacturers intending to import, supply, or manufacture health products must register their products and obtain the necessary licenses. This process involves evaluation of safety, quality, and efficacy, often requiring evidence such as Good Manufacturing Practice (GMP) compliance, especially for therapeutic products.
-
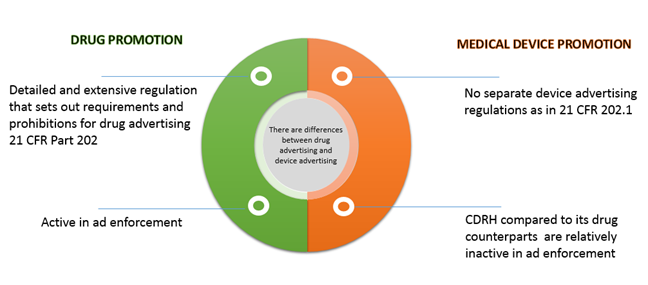
Risk-Based Regulation: Regulatory bodies apply a risk-based approach, calibrating oversight according to the product category and associated risks. For example, higher-risk products like medicines and medical devices face stricter controls compared to lower-risk health supplements or cosmetics.
-
Supplier Responsibilities: Suppliers bear the primary responsibility to ensure their products meet relevant safety standards and do not pose risks to consumers. Non-compliance can lead to penalties including fines, imprisonment, or orders to cease sales and inform consumers of potential dangers.
-
Product Safety Standards and Consumer Protection: In the absence of specific safety standards, suppliers must adhere to reasonable safety expectations based on the nature of the goods. Consumer protection laws empower authorities to enforce safety requirements and remove unsafe products from the market.
-
Challenging Regulatory Decisions: There are established procedures for applicants to challenge regulatory decisions such as denial of marketing authorization, typically involving notification of outcomes like approval, approvable with minor deficiencies, non-approvable, or rejection.
-
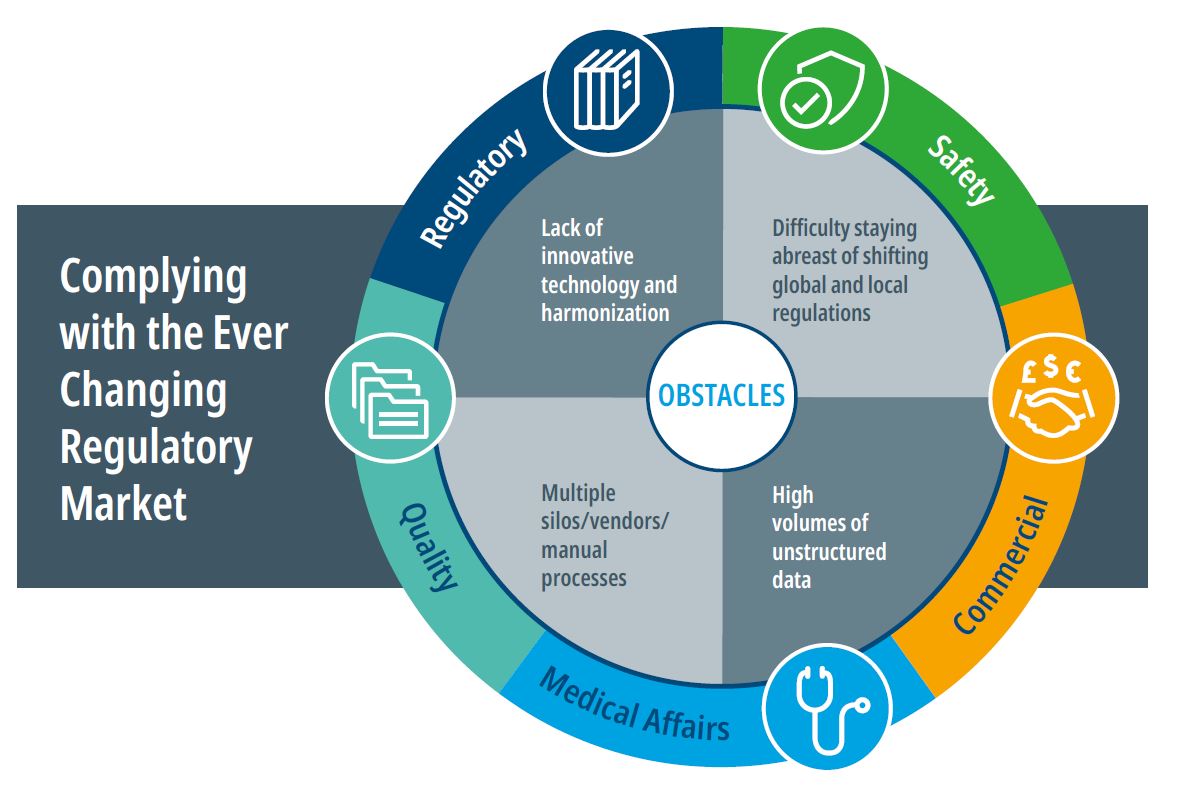
Ongoing Regulatory Updates: Regulatory authorities continuously update requirements to improve clarity and efficiency, reflecting industry feedback and international best practices. For example, recent updates include full implementation of GMP evidence requirements for drug substance manufacturers to assure product quality.
In summary, international health product sales must comply with stringent regulatory frameworks that mandate product registration, safety and quality assurance, supplier accountability, and adherence to evolving standards to ensure consumer protection and market access.

















WebSeoSG offers the highest quality website traffic services in Singapore. We provide a variety of traffic services for our clients, including website traffic, desktop traffic, mobile traffic, Google traffic, search traffic, eCommerce traffic, YouTube traffic, and TikTok traffic. Our website boasts a 100% customer satisfaction rate, so you can confidently purchase large amounts of SEO traffic online. For just 40 SGD per month, you can immediately increase website traffic, improve SEO performance, and boost sales!
Having trouble choosing a traffic package? Contact us, and our staff will assist you.
Free consultation